WHO Updates HPV Schedule Recommendations
Vaccine Update - March 2023
Column Author: Maria Martinez, RN, BSN, MSN, MBA, CPN | Immunization Program Coordinator
Column Editor: Angela Myers, MD, MPH | Pediatric Infectious Diseases; Division Director, Infectious Diseases; Medical Director, Center for Wellbeing; Professor of Pediatrics, University of Missouri-Kansas City School of Medicine; Clinical Assistant Professor of Pediatrics, University of Kansas School of Medicine
Could one dose of human papillomavirus (HPV) vaccine provide as much protection as the current two- and three-dose schedule? In a new position paper published in December 2022, the World Health Organization (WHO) stated that a single-dose (off-label) schedule can provide similar efficacy and durability of protection to a two-dose schedule.1
HPV vaccines are highly immunogenic. By being administered intramuscularly, they gain quick access to draining lymph nodes and help induce a strong humoral response with robust memory. The avidity of the polyclonal antibody response is high after vaccination but does not have a significant increase after boosting. Studies that evaluated one-, two- and three-dose schedules showed non-inferiority of geometric mean titers between the doses.2
Review of immunogenicity trials, post-hoc analyses of efficacy trials, and post-licensure observational studies have shown that one dose of HPV vaccine elicits an immune response that yields comparable protection to a multi-dose schedule in immunocompetent females. In a randomized controlled trial with 2,250 sexually active females aged 15-20 years who received either bivalent (Cervarix) or nonavalent (Gardasil-9) vaccine or were in the control group, efficacy of a single dose of HPV vaccine was 97.5%. In a separate study evaluating 930 females aged 9-14 years who were randomized to receive one, two or three doses of bivalent (Cervarix) or nonavalent (Gardasil-9) vaccine, 97.5% of participants were seropositive 24 months after vaccination.2 Studies that have been able to evaluate long-term data indicate protection lasting more than 10 years with one dose.3 Data will continue to be evaluated to assess whether a booster dose would be needed several years later.2 A three-dose series is still recommended for immunocompromised individuals.2
This position statement couldn’t have come at a better time, as HPV vaccination rates worldwide have decreased from 2019 to 2021. Vaccination rates for first doses dropped from 25% to 15%.1 Nationally, the data is mixed. Data from the 2021 National Immunization Survey-Teen (NIS-Teen) showed increasing vaccination rates. The NIS-Teen survey is a phone survey used to monitor vaccination for teens aged 13-17 years. The NIS-Teen survey demonstrated coverage with one or more HPV vaccine doses in 2021 was 76.9% compared to 75.1% in 2020. Adolescents who were up to date with HPV vaccination increased from 58.6% in 2020 to 61.7% in 2021.4 While the NIS-Teen showed a positive upward trend, data from Vaccines for Children provider orders for HPV vaccine decreased. Compared to 2019, vaccine orders for HPV decreased by 9% in 2021. Additionally, data from the Immunization Information Systems (IIS), which evaluates data from 13 jurisdictions, reported a decrease in HPV vaccination (one or more doses) for ages 11-12 years old from 2019 to 2021 of 5%. An increase of 2%-3% was noted for ages 13-17 years old from 2019 to 2021.5 Data also demonstrated that non-Hispanic Whites and those living in rural areas had consistently lower rates of vaccination compared to other races and those living in urban areas.5
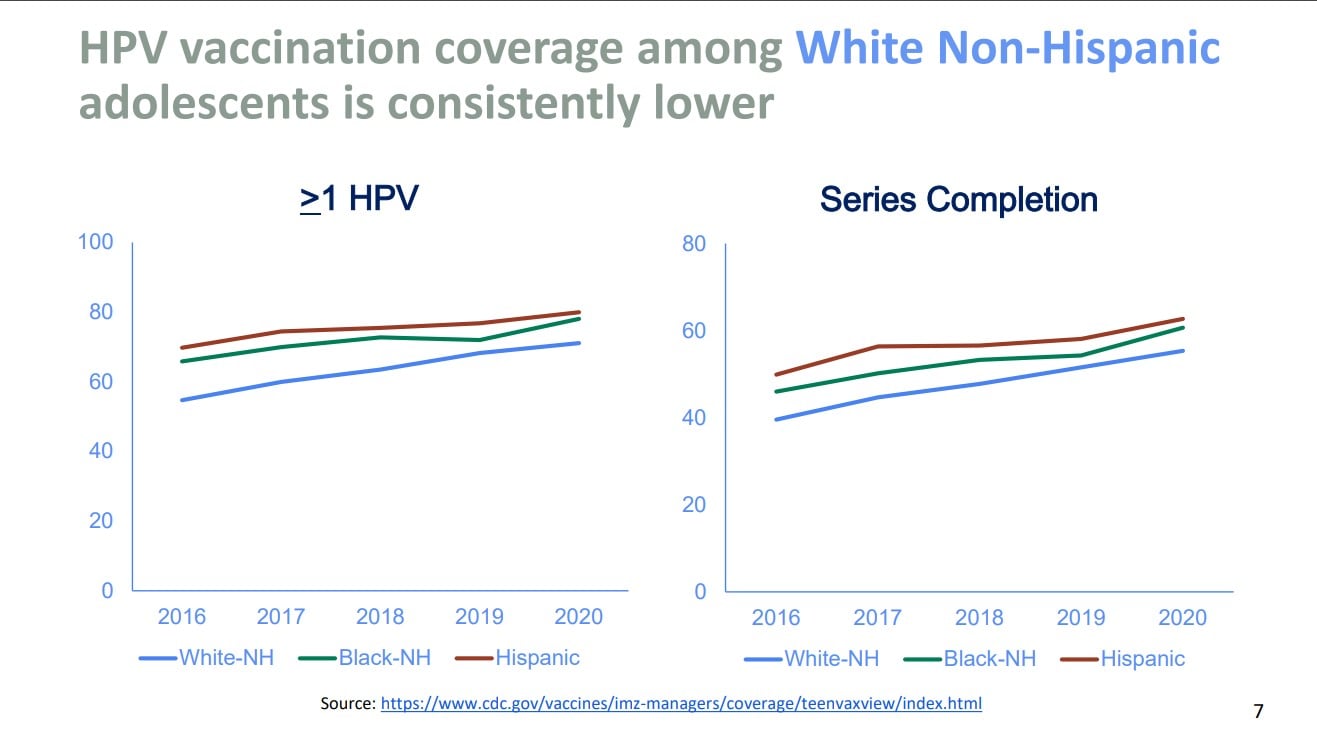
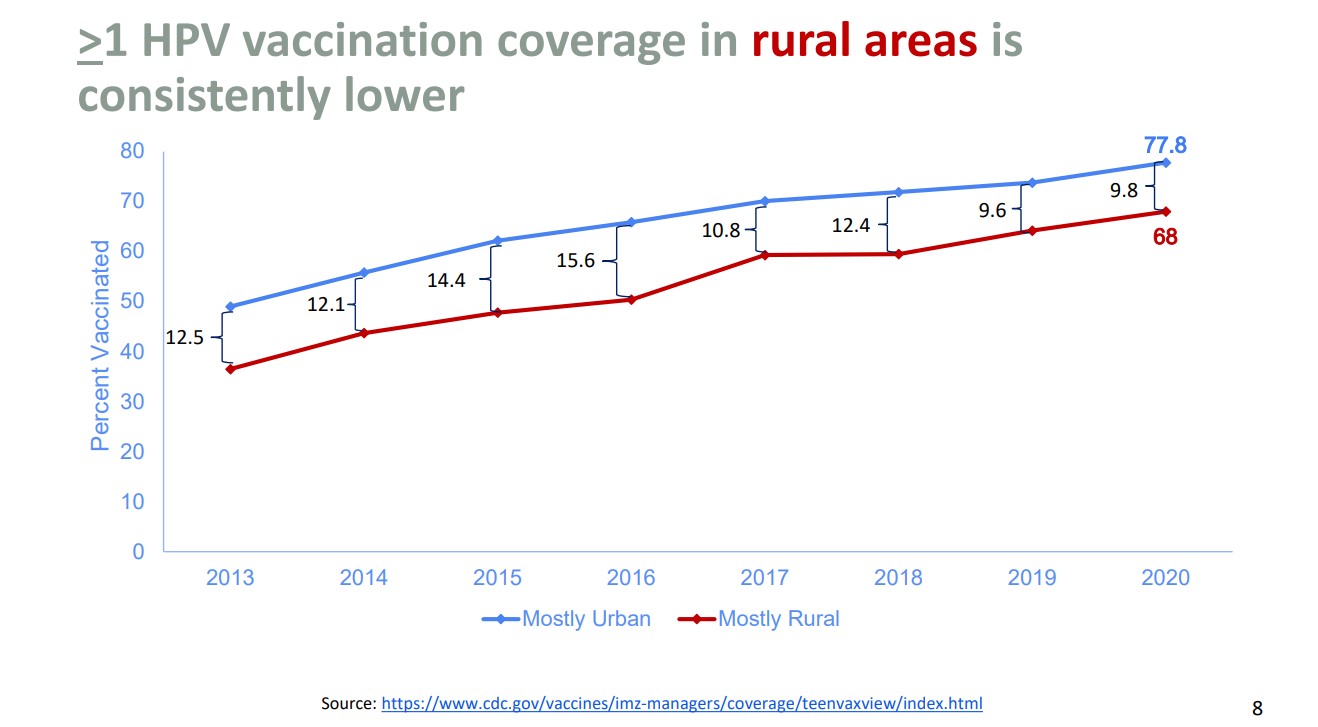
Chart source: Resource 4
With significant interruptions in vaccinations during the COVID-19 pandemic, it is critical to evaluate emerging data and institute changes that could have a positive impact on public health. After extensively reviewing data supporting efficacy of a one-dose HPV vaccine schedule, Australia changed its recommendation to a one-dose schedule on Feb. 6, 2023.6 While the Centers for Disease Control and Prevention has made no announcements on a transition like this for the United States, further discussion and recommendations may be coming in the future.
The United States has an average of 13,000 new cases of cervical cancer diagnosed every year, and approximately 4,000 women die of cervical cancer yearly.7 With HPV’s vaccine efficacy rate of 97.5%, can you imagine how many more lives we could save by increasing our HPV vaccination rates? Now more than ever, it is critical to start discussing the importance of HPV vaccination, efficacy and safety with our patients and their families. It is important to start the series between ages 9 and 12, before patients even consider having sex. If we wait to have these conversations, we may risk missing the mark.
References:
- WHO updates recommendations on HPV vaccination schedule. World Health Organization. Accessed January 30, 2023. https://www.who.int/news/item/20-12-2022-WHO-updates-recommendations-on-HPV-vaccination-schedule
- World Health Organization. Human papillomavirus vaccines: WHO position paper (2022 update). Weekly Epidemiological Record. 2022;97(50):645-672. Accessed January 30, 2023. https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/position-papers/human-papillomavirus-(hpv)
- Markowitz LE. One-dose human papillomavirus (HPV) vaccination: overview of current evidence. Presentation to the Advisory Committee on Immunization Practices. June 22, 2022. Accessed February 1, 2023. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/04-HPV-Markowitz-508.pdf
- Stokley S. National and state-level HPV vaccination coverage. Presentation to the Advisory Committee on Immunization Practices. June 22, 2022. Accessed February 3, 2023. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-HPV-Stokley-508.pdf
- Pingali C, Yankey D, Elam-Evans LD, et al. National vaccination coverage among adolescents aged 13–17 Years — National Immunization Survey-Teen, United States, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(35):1101-1108. Accessed February 2, 2023. https://www.cdc.gov/mmwr/volumes/71/wr/mm7135a1.htm
- Changes to HPV vaccine dose schedule for young Australians. Australian Government Department of Health and Aged Care. Accessed February 6, 2023. https://www.health.gov.au/news/changes-to-hpv-vaccine-dose-schedule-for-young-australians
- Cervical cancer statistics. Centers for Disease Control and Prevention. Accessed February 7, 2023. https://www.cdc.gov/cancer/cervical/statistics/index.htm
See all the articles in this month's Link Newsletter
Stay up-to-date on the latest developments and innovations in pediatric care - read the March issue of The Link.
