Don't Forget About MenB
Vaccine Update - June 2023
Column Author: Maria Martinez, RN, BSN, MSN, MBA, CPN | Immunization Program Coordinator
Column Editor: Angela Myers, MD, MPH | Pediatric Infectious Diseases; Division Director, Infectious Diseases; Medical Director, Center for Wellbeing; Professor of Pediatrics, University of Missouri-Kansas City School of Medicine; Clinical Assistant Professor of Pediatrics, University of Kansas School of Medicine
MenB vaccines have the lowest administration rates of the vaccines available for adolescents in the United States. Data from the National Immunization Survey-Teen for 2021 report MenB vaccination rates with one or more doses administered were a staggering 31.4%. Tdap administration rates with one or more doses were 89.6%, MenACWY administration rates with ≥ one doses were 89.0% and HPV administration rates with one or more doses were 76.9%.1 With rates for MenB so low, are we doing everything we can as providers to promote this vaccine and protect our patients? 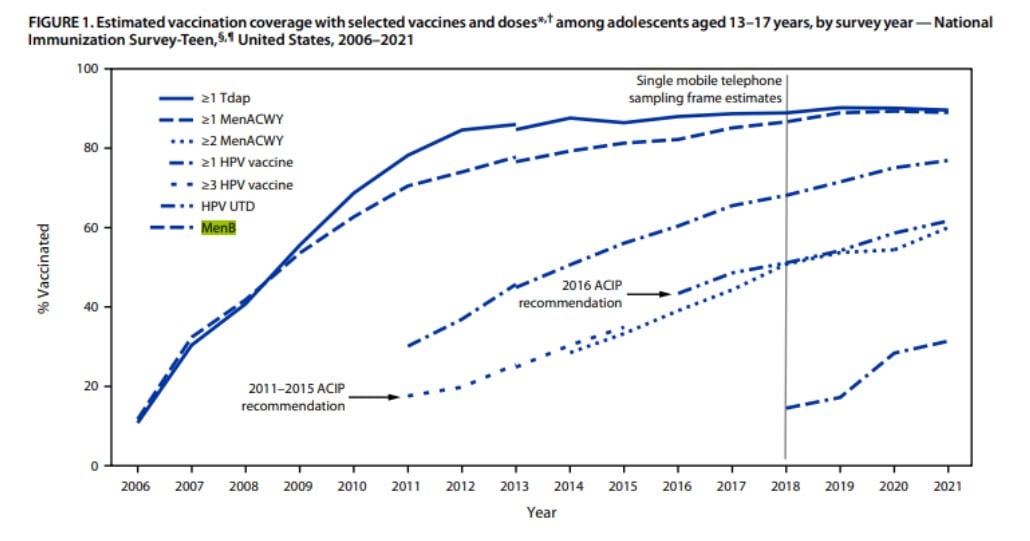
Meningococcal disease is caused by the bacteria Neisseria meningitidis and clinically presents as meningitis (50%), septicemia (35%-40%) or, less commonly, pneumonia (10%).4 Transmission occurs from person to person (patients or asymptomatic carriers) through droplets of respiratory secretions (e.g., by coughing, kissing or sharing eating utensils).4,5 Disease can be very severe with a case-fatality ratio in the U.S. of 15%, and is slightly higher in older adolescents and older adults. Additionally, 10%-20% of survivors suffer long-term complications such as neurologic disability, limb or digit loss, and hearing loss. Of the 12 serogroups for Neisseria meningitidis, serogroups A, B, C, W, X and Y cause the most invasive disease.4 Serogroup B has the highest number of incidences out of serogroups A, B, C, W, X and Y.6
MenB vaccine was first introduced in the United States at the end of 2014. Initial recommendations for MenB were for young adults aged 16 through 23 years with the preferred age for vaccination being 16-18 years of age (recommendation Category B). Category B recommendation indicated individual clinical decision-making.2 However, in 2019, the Advisory Committee on Immunization Practices (ACIP) changed its recommendation from “Category B” to “shared clinical decision-making.” This change was made to provide more clarity about the need to inform the patient of the option to be vaccinated against meningococcal serogroup B disease and to emphasize that the decision should be made by the provider and patient together.3
The change in recommendation from “Category B” to “shared clinical decision-making” came during the rush/whirlwind of health care challenges facing us during the COVID-19 pandemic. With many health care providers and health care systems struggling to stay afloat, did this updated recommendation go unnoticed by many? Another possible factor in the low vaccination rates could be immunization forecasters within electronic health records (EHRs) failing to note when the vaccine is due for age-eligible patients. Many health care providers rely on immunization forecaster systems to help guide them with recommended patient vaccinations. Since the Centers for Disease Control and Prevention (CDC) has this vaccine listed as “shared clinical decision-making,” EHR systems that rely on CDC recommendations may not be alerting health care providers that patients are eligible for MenB. Could these be attributing factors for the low MenB vaccination rates?
A recent study comparing meningococcal serogroup B vaccination rates among adolescents at four diverse outpatient clinics found that 32% of patients received the full meningococcal serogroup B vaccine series. The study evaluated rates from two pediatric clinics and two family medicine clinics. Most of the patients who completed their MenB series were seen in a pediatric clinic. The findings in this study strongly indicate the need for provider education regarding current MenB vaccination recommendations.7
Health care providers need to make sure they are knowledgeable about the most up-to-date vaccine recommendations and start the discussion with parents on their recommendation for initiation for MenB vaccine. A category of “shared clinical decision-making” does not mean that a vaccine is any less important. Providers should give a strong recommendation and educate their patients and families on the serious risk of complications for patients if unvaccinated against MenB. With shared clinical decision-making, the following should be considered for patients aged 16-23 who are not at increased risk for meningococcal disease.4
- While uncommon, with an average of 20-50 cases among 16-23 years of age per year, serogroup B meningococcal disease is very serious with a high risk of death and significant long-lasting complications.
- College students face an increased risk, especially those going on campus for the first time, attending a four-year college, living in any type of college housing or participating in sororities and fraternities. (In 2022, 62% of 2022 high school graduates were enrolled in colleges or universities.8)
- MenB vaccines are safe and effective against most strains of meningococcal serogroup B bacteria but provide protection for only one to two years.3,4
As health care providers, we are the most trusted source for medical information, making it our responsibility to educate our families on vaccine-preventable diseases. Our patients cannot be fully protected if we fail to educate and recommend due to systems barriers that rely solely on clinician memory. Creation of clinical alerts that remind us of a patient’s eligibility is one way to help increase the vaccination rate for this important vaccine.
References:
- Pingali C, Yankey D, Elam-Evans LD, et al. National vaccination coverage among adolescents aged 13–17 years — National Immunization Survey-Teen, United States, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(35):1101-1108. https://www.cdc.gov/mmwr/volumes/71/wr/pdfs/mm7135a1-h.pdf
- Advisory Committee on Immunization Practices (ACIP). Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for use of serogroup B meningococcal (MenB) vaccines in adolescents and young adults (including college students). Centers for Disease Control and Prevention. Last reviewed October 21, 2015. Accessed May 24, 2023. https://www.cdc.gov/vaccines/acip/recs/grade/menb-young-adults.html
- Wexler, D. Technically speaking: ACIP updates its guidance on the use of HPV, PCV13, HepA, HPV, and MenB vaccines at June 26–27 meeting. Immunize.org. Updated August 13, 2019. Accessed May 25, 2023. https://www.immunize.org/technically-speaking/20190725.asp
- Ask the experts: Meningococcal B. Immunize.org. Updated April 15, 2021. Accessed May 24, 2023. https://www.immunize.org/askexperts/experts_meningococcal_b.asp
- First vaccine approved by FDA to prevent serogroup B meningococcal disease. News release. U.S. Food and Drug Administration. October 29, 2014. Accessed May 25, 2023. https://www.fda.gov/news-events/press-announcements/first-vaccine-approved-fda-prevent-serogroup-b-meningococcal-disease
- Meningococcal disease surveillance. Centers for Disease Control and Prevention. Last reviewed March 6, 2023. Accessed May 25, 2023. https://www.cdc.gov/meningococcal/surveillance/index.html
- Comparison of adolescent meningococcal B vaccination rates in four diverse outpatient clinics. J Pediatr Pharmacol Ther. 2022;27(8):703-706. doi:10.5863/1551-6776-27.8.703
- College enrollment and work activity of recent high school and college graduates summary. News release. U.S. Bureau of Labor Statistics. Last modified April 26, 2023. Accessed May 25, 2023. https://www.bls.gov/news.release/hsgec.nr0.htm
See all the articles in this month's Link Newsletter
Stay up-to-date on the latest developments and innovations in pediatric care – read the June issue of The Link.