Outbreaks, Alerts and Hot Topics
December 2022
A Great Surge: Influenza, RSV and COVID-19

Author and Column Editor: Chris Day, MD | Pediatric Infectious Diseases | Director, Transplant Infectious Disease Services; Medical Director, Travel Medicine | Assistant Professor of Pediatrics, University of Missouri-Kansas City School of Medicine; Clinical Assistant Professor of Pediatrics, University of Kansas School of Medicine
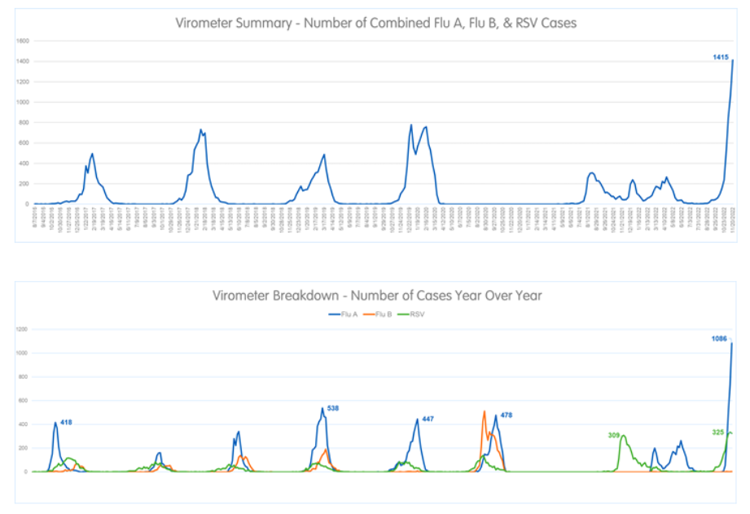
In most of the United States, levels of reported influenza-like illness (ILI) are considered by the Centers for Disease Control and Prevention (CDC) to be high or very high as of the most recent week reported (Figure). CDC estimates that this respiratory season has seen 120,000 hospitalizations from influenza, the highest cumulative total for this week of the year since 2010-2011, including the 25,906 patients admitted this past week. Thirty pediatric influenza deaths have been reported so far this season.1 RSV (respiratory syncytial virus) continues to circulate at high levels both nationally and in our region,2 and COVID-19 cases are on the rise with 458,986 cases reported in the most recent week available (the prior week had 306,733 cases reported).3 Concerns for a “tripledemic” of the three viruses were raised in the popular press and by influenza experts at least as early as October based on an early and severe influenza season in the Southern Hemisphere and an early rise in influenza cases in the United States.4 Respiratory testing data from Children’s Mercy Kansas City shows that positive tests for influenza far exceed what we have seen in recent seasons (Figure 2a and 2b).
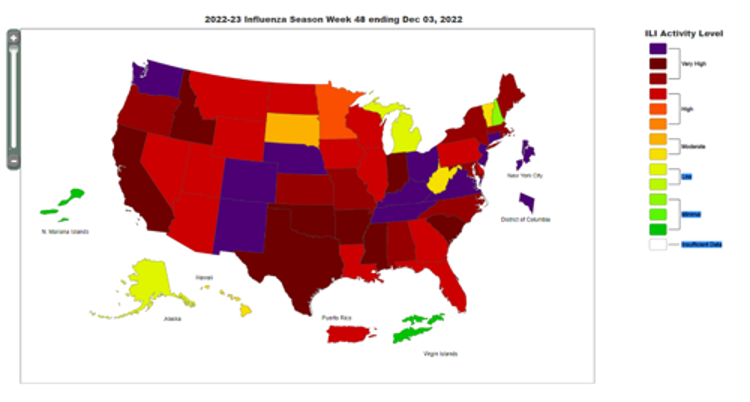
Figure 1: CDC Weekly U.S. influenza surveillance report.1
Figures 2a and 2b: Children's Mercy Kansas City Weekly Virometer Reports
Individuals can still benefit from immunization against two of these viruses. CDC reports that the majority of circulating influenza viruses are antigenically similar to those strains included in this season’s influenza vaccine. These strains are predominately H3N2 influenza A (76%), but H1N1 (24%) is circulating as well. Even after an influenza A infection, there is benefit to immunization to prevent not only morbidity from influenza B, but to prevent morbidity from the other influenza A strain.1 SARS-CoV-2 lineages that appear to be dominant now include BA.5, BQ.1 and BQ.1.1. These and most other currently circulating SARS-CoV-2 in the United States continue to be Omicron variant viruses.5 Both primary series and boosters against COVID-19 remain crucial for mitigating the impact of these viruses, but, as of October, only 68.8% of the U.S. population had completed a primary series and 48.3% of the booster-eligible population had not received a booster dose.6 It remains important to encourage influenza and COVID-19 vaccination both to protect individual patients and to reduce burdens on the health care system.
Treatment recommendations for influenza are complicated by current, localized shortages of oseltamivir. CDC recently issued a Health Alert Network advisory on prioritizing oseltamivir and other influenza antivirals that cannot be fully summarized here. Briefly, in the setting of an oseltamivir shortage:
- Testing patients for influenza (or having a positive test in a household contact) becomes more important for making decisions about antiviral therapy.
- Hospitalized patients should have highest priority for oseltamivir treatment as the evidence of benefit for antivirals in this population has been with oseltamivir. Antiviral treatment should be started as soon as possible for any patient hospitalized with influenza.
- Outpatients that should be most prioritized for antiviral treatment are those who can be treated within 48 hours of illness onset AND are at greatest risk for complications of influenza (including children under age 2).
- Oseltamivir is the only recommended oral antiviral for children under 5. If suspension is unavailable, a suspension compounded from capsules can be requested from pharmacies.7
Baloxavir is now an option as a single-dose treatment for healthy children weighing more than 20 kg (pills cannot be split or crushed) to as young as age 5 as well as for many children age 12 and up who are at high risk of developing influenza complications. Baloxavir is not recommended for immunocompromised patients. Inhaled zanamivir or IV peramivir are also appropriate for some pediatric patients. Recall that zanamivir is contraindicated in people with underlying respiratory disease due to risk of bronchospasm.8
References:
- Weekly U.S. influenza surveillance report. Centers for Disease Control and Prevention. December 9, 2022. Accessed December 9, 2022. https://www.cdc.gov/flu/weekly/index.htm
- National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Respiratory syncytial virus (RSV) surveillance. Centers for Disease Control and Prevention. December 7, 2022. Accessed December 9, 2022. https://www.cdc.gov/surveillance/nrevss/rsv/index.html
- Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. December 9, 2022. Accessed December 9, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_weeklycases_select_00
- Mandavilli A. A ‘tripledemic’? Flu, R.S.V. and Covid may collide this winter, experts say. New York Times. October 27, 2022. https://www.nytimes.com/2022/10/23/health/flu-covid-risk.html
- COVID data tracker. Centers for Disease Control and Prevention. December 12, 2022. Accessed December 12, 2022. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. COVID data tracker weekly review, interpretive summary for December 2, 2022. Centers for Disease Control and Prevention. December 2, 2022. Accessed December 12, 2022. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/past-reports/12022022.html
- Center for Preparedness and Response (CPR). Interim guidance for clinicians to prioritize antiviral treatment of influenza in the setting of reduced availability of oseltamivir. Centers for Disease Control and Prevention. December 14, 2022. Accessed December 15, 2022. https://emergency.cdc.gov/han/2022/han00482.asp
- National Center for Immunization and Respiratory Diseases (NCIRD). Influenza antiviral medications: summary for clinicians. Centers for Disease Control and Prevention. September 9, 2022. Accessed December 12, 2022. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm