Wide World of Vaccines
June 2021
mRNA Vaccines vs. Variants, but the Variants’ Names “are all Greek to me”

Column Editor: Christopher Harrison, MD | Professor of Pediatrics, UMKC School of Medicine | Clinical Professor of Pediatrics, University of Kansas School of Medicine
The good pandemic news is that U.S. new cases, hospitalizations and deaths have declined to Spring 2020 levels, seemingly due to ~55% of U.S. adults being fully immunized with an mRNA vaccine (Pfizer or Moderna) and increasing mRNA vaccination (Pfizer) of adolescents. Adding 55% for vaccine-induced immunity to the, at least partial, immunity in the 10-15% who recovered from SARS-CoV-2 infection, we are near the 70% needed for “herd immunity.” So, the U.S. is opening up and news should remain good unless one or more variant (e.g., UK B.1.1.7, Brazilian P.1/P.2, South African B.1.351 or India B.1.617.2 virus) becomes more prevalent and starts escaping either vaccine-induced (not very likely given current data) or infection-induced immunity (possible in light of real-world outbreaks internationally).
Variants are the elephant in the room because they have wreaked havoc among populations that had been estimated to have or be close to having infection-induced herd immunity, mostly mild or asymptomatic infections with the original Wuhan virus. But we are seeing that new variants can infect patients who recovered from original SARS-CoV-2 virus infections, and these breakthroughs can be severe – particularly if the original infection produced no or mild symptoms (e.g., India and Brazil). Interestingly, hospitalized but recovered patients appear to be protected against severe variant disease.
Now two U.S. variants (the New York B.1.526 virus and the California B.1.429 virus) are emerging. These also raise worries that we might not be as protected by vaccine or prior infections because they also have occurred in populations that had high rates of original Wuhan virus infection (California and New York).
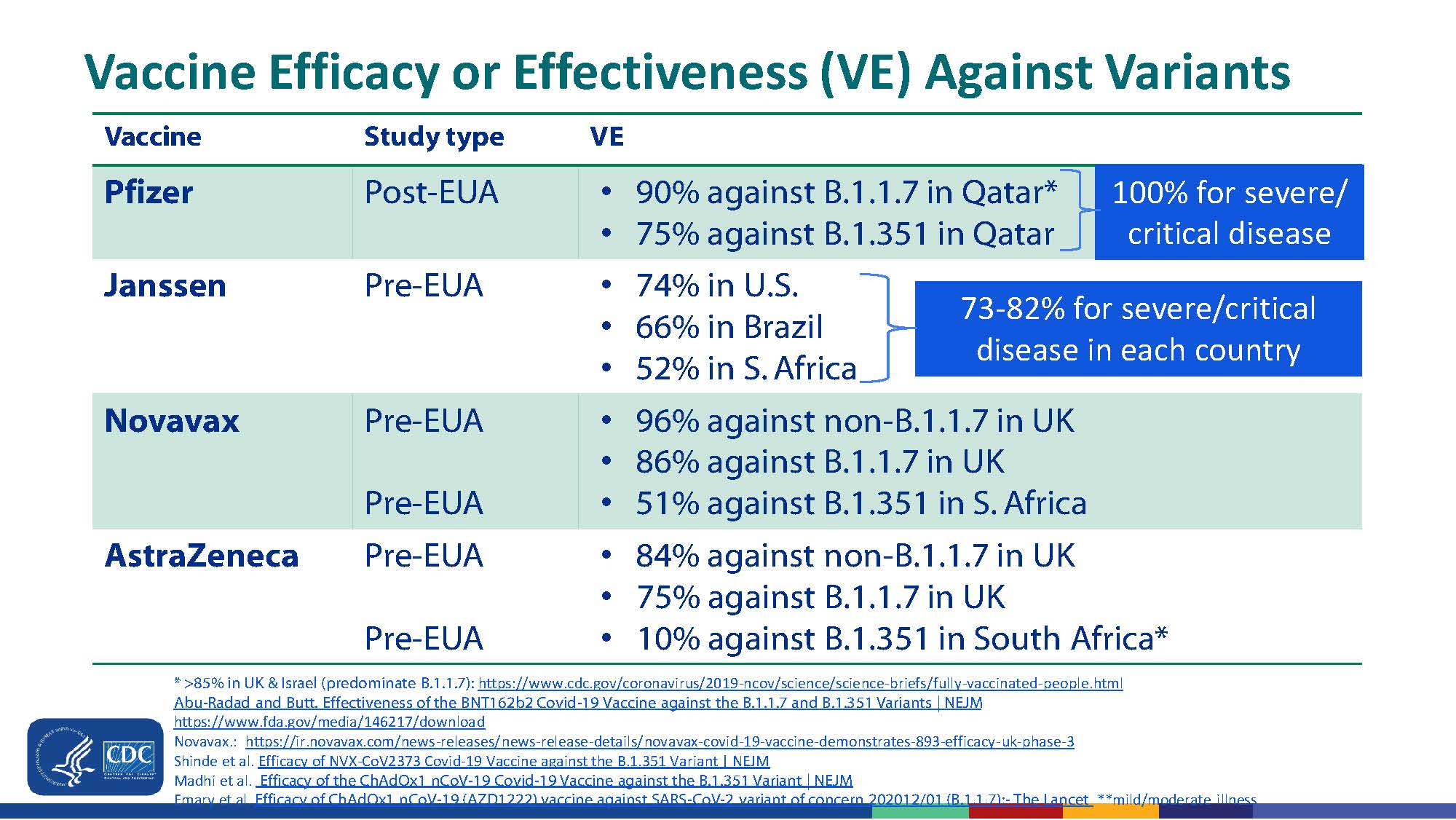
So how well do vaccines protect against variants originating outside or inside the U.S.? This is really important given the increasing numbers of variant infections in the U.S. these past months. Table 1 lists available clinical data (courtesy of CDC). The mRNA vaccines seem to be as effective against the U.K. variant, the California variant and the New York variant as against the original SARS-CoV-2 virus. Importantly, this seems to translate into high protection (>90%) against hospitalization or severe symptomatic disease. Similarly, mRNA vaccines have somewhat lower protection against infection due to South African virus but have comparably high protection against moderate to severe disease.
Finally, data from in vitro assays indicates that mRNA-induced antibodies neutralize the Brazilian and India viruses, although 6- to 8-fold higher titers are required to neutralize Brazilian P.1 and P.2 viruses and the India variants. Unpublished sparse clinical data suggest that mRNA vaccines also protect against moderate to severe disease due to S. African and India variants, at least in the first months after the second dose.
Non-mRNA vaccines: Astra-Zeneca vaccine induces minimal and Johnson & Johnson vaccine induces less neutralizing antibody to the variant viruses other than the U.K. variant, paralleling clinical trial data to date indicating little protection for Astra-Zeneca’s and moderate protection for the Johnson & Johnson vaccine vs. the South African and Brazilian viruses. In contrast, the pending Novovax vaccine’s induced antibodies seem comparable to mRNA vaccines in neutralizing capabilities.
So, the almost exclusive U.S. use of mRNA vaccines predicts continued improvement in the pandemic on a better trajectory than we expected six months ago. This should allow further normalizing of activities, as long as highly unimmunized pockets of people do not allow emergence of a new more immune-evasive variant.
Bottom line: mRNA vaccine-induced immunity seems, to date, better than infection-induced immunity at protecting against variants so we need to continue to push for higher rates of vaccine uptake. And as the vaccine is authorized for younger children by the fourth quarter of 2021, we will add an extra layer to the increasing degree of herd immunity.
Greek Letters?
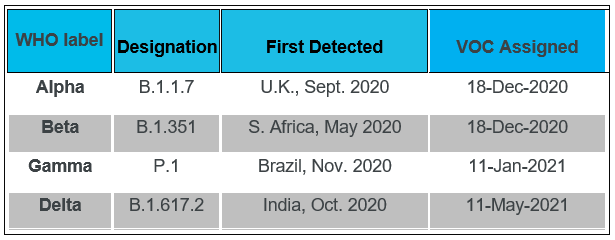
In the first week of June the World Health Organization (WHO) assigned Greek Letters to variants in order to “simplify” nomenclature, allow easier discussion of variants for non-scientists, and remove stigma from the countries where the variants were first derived. Variants of concern (VOC – those with evidence of increased transmissibility, more severe disease, significant reduction in neutralization by antibodies, reduced effectiveness of treatments or vaccines, or diagnostic detection failures) and variants of interest (VOI – those with genetic markers changing receptor binding, reduced antibody neutralization, reduced treatment efficacy, potential diagnostic impact, or predicted increased transmissibility or severity) are listed in Table 2. So, when you hear someone talk about delta virus, they are talking about the India B.1.617.2 variant. Now the question is, “Do the Greek letters really make it easier?” Only time will tell.
Table 1. Vaccine efficacy or effectiveness (VE) for SARS-CoV-2 variants from clinical data, excluding India variants.*
Source: CDC. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/10-COVID-Scobie-508.pdf (Slide 22)
* VE data on Moderna mRNA vaccine VE against the variants is not yet published but there have been hints in press release information that it is similar to Pfizer mRNA vaccine.
Table 2. Greek letter designation for variants of concern and interest
Variants of Concern (VOC)
Variants of Interest (VOI)
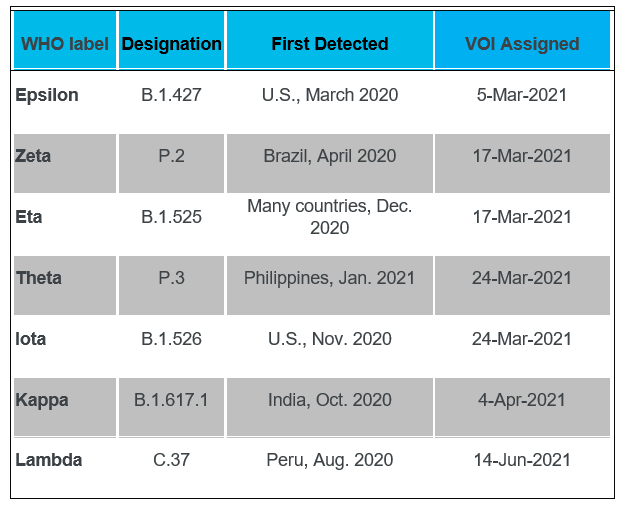
Data Source: WHO. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
References:
- Sabino EC, Buss LF, Carvalho MPS, Prete CA, Crispim MAE, Fraiji NA, Pereira RH, Parag KV, da Silva Peixoto P, Kraemer MU. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397:452–455. doi: 10.1016/S0140-6736(21)00183-5.
- Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 variants'. N Engl J Med. 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2104974.
- Liu Y, et al. BNT162b2-elicited neutralization against new SARS-CoV-2 spike variants. N Engl J Med. May 12, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2106083.
- Abdool Karim SS, de Oliveira T. New SARS-CoV-2 variants - clinical, public health, and vaccine implications. N Engl J Med. 2021;384(19):1866-1868.
- Fabiani M, Margiotti K, Viola A, Mesoraca A, Giorlandino C. Mild Symptomatic SARS-CoV-2 P.1 (B.1.1.28) infection in a fully vaccinated 83-year-old man. Pathogens. 2021;10(5):614. Published 2021 May 17. doi:10.3390/pathogens10050614.
