Vaccine Update: What Can We Expect for Fall Vaccines
Column Author: Maria Martinez RN, BSN, MSN, MBA, CPN
Column Editor: Angela Myers, MD, MPH | Chief Wellbeing Officer, Center for Wellbeing, Professor of Pediatrics, University of Missouri-Kansas City School of Medicine; Clinical Assistant Professor of Pediatrics, University of Kansas School of Medicine
In early June, after all 17 Advisory Committee on Immunization Practices (ACIP) members were replaced with eight new members, several of whom are vaccine skeptics, many medical professionals were left concerned for the future of vaccine policy. Later that month, over 4,000 people logged on to watch the livestream of the June ACIP meeting.
Until recently, ACIP was an independent panel of some of the leading vaccine experts in the United States. These vaccine experts were selected after being fully vetted by the Department of Health and Human Services through a lengthy process. They worked closely with many liaison organizations such as the American Academy of Pediatrics, American Academy of Family Physicians, American College of Obstetricians and Gynecologists, and the American Medical Association to develop immunization recommendations. They convened three times a year to hear Centers for Disease Control and Prevention (CDC) scientists, Food and Drug Administration (FDA) experts and vaccine manufacturers present data on clinical trials, disease burden and post-marketing surveillance. Based on the science-based evidence that was presented, ACIP members would vote on who was eligible for a vaccine product and when it should be administered. These decisions would provide guidance to physicians, advanced practice providers, pharmacists, insurance companies and public health agencies.
The June ACIP meeting agenda was shortened from what was originally planned and last-minute changes to the agenda were incorporated. As we gear up for the fall, below is what was discussed for influenza, respiratory syncytial virus (RSV) and COVID-19 immunizations:
The ACIP voted to approve influenza vaccines for the fall with the caveat that thimerosal be removed from the limited supply (5% of influenza vaccines) that has it as an ingredient. Thimerosal is used as a preservative in multi-dose vials of influenza vaccine to prevent bacterial and fungal growth. Inaccurate and misleading information was presented at the ACIP meeting by a new ACIP member regarding the safety of thimerosal. Thimerosal contains small amounts of ethylmercury, which is safe and the body is able to quickly excrete it. Decades of studies have proven that thimerosal is safe in the quantities used in vaccines. Due to its safety profile, thimerosal has also been used in drugs and contact solutions since the 1930s. It is easy for people to assume that ethylmercury could be toxic given the similarity in name to methylmercury. Methylmercury is found in many fish and can accumulate in the human body, causing harm at high levels.1
A new RSV long-acting monoclonal antibody for infants, clesrovimab, was approved by the ACIP. Clesrovimab will be available in a single-dose prefilled syringe (105 mg/0.7 ml). The dose will be the same for all infants regardless of weight. Clesrovimab and nirsevimab recommendations are the same for infants under 8 months of age born during or entering their first RSV season: one dose given at birth or shortly after. Both products are effective with no preferential recommendation between one or the other. Nirsevimab, however, is the only product that can be administered for a second RSV season to children 8 through 19 months of age who are at increased risk for severe RSV disease. High-risk infants eligible for a second season dose of nirsevimab could have received nirsevimab or clesrovimab for their first RSV season. Data regarding RSV immunization effectiveness, uptake and impact for the 2024-2025 RSV season demonstrated significant positive outcomes. Nirsevimab led to decreased RSV-associated emergency department visits, hospitalization and critical illness for infants experiencing their first RSV season. Similar outcomes were noted for infants born to mothers who received maternal RSV vaccine. Uptake data showed that 57% of infants were either born to a mother who received maternal RSV vaccine or received nirsevimab. When compared to infants prior to the introduction of RSV immunization, RSV-associated hospitalizations among eligible infants were reduced by 30%-40% and by up to 50% in infants under 2 months of age.2 Providing immunization to infants under age 8 months of age targets the population most likely to have RSV-associated hospitalizations. (Figure 1)
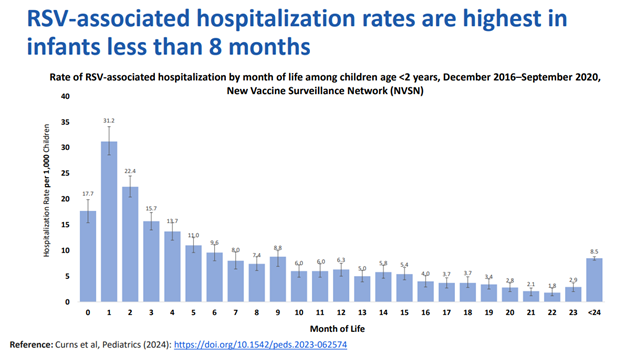
Fig. 1- Respiratory Syncytial Virus-Associated Hospitalizations Among Children <5 Years Old: 2016 to 2020
Data shared regarding COVID-19 vaccines reaffirms that they continue to be safe and effective. COVID-19 continues to circulate year-round with summer and winter peaks. In the pediatric population, most hospitalizations and deaths occurred in children younger than 2 years, with most having no underlying health conditions. Children younger than age 1 also had higher rates of hospitalization from COVID-19 than from influenza. (Figure 2) Since infants younger than 6 months cannot be vaccinated, the best way to protect them is through maternal vaccination. Of the COVID-19 vaccine-eligible children and adolescents who were hospitalized with COVID-19, 89% of them had no record of receiving the most recent COVID-19 vaccine. Not only are hospitalizations and deaths concerning, long COVID is also affecting pediatric patients, with estimates from 2023 indicating over 300,000 pediatric patients experience long COVID.3
In the adult population, adults aged 65 and older had the highest rates of hospitalization, comprising 72% of COVID-19-associated hospitalizations — 65% of them had not received a dose of 2024-2025 COVID-19 vaccine. (Figure 3) In pregnant women admitted for COVID-19-related symptoms between April 2024 and March 2025, half did not have underlying health conditions, and 92% of them had not received a dose of COVID-19 vaccination since July 1, 2023.3
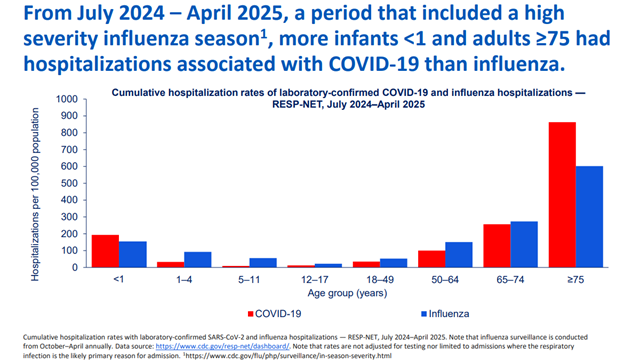
Fig. 2 - Current Epidemiology of COVID-19
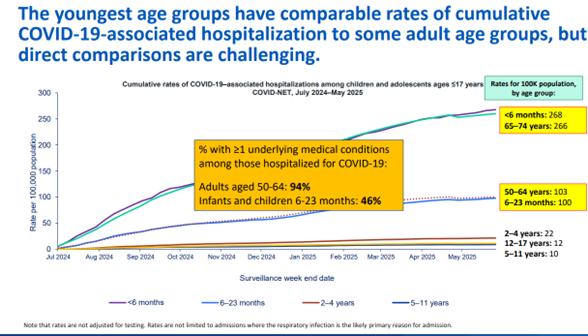
Fig. 3 - Current Epidemiology of COVID-19
The COVID-19 viruses currently circulating come from the JN.1 lineage. The current viruses were able to be neutralized with sera from individuals who received the 2024-2025 COVID-19 vaccine. At the FDA’s Vaccine and Related Biological Products Advisory Committee (VRBPAC) meeting in May, genomic and phenotypic data was reviewed and the committee unanimously voted to recommend a monovalent JN.1 lineage vaccine. Vaccine manufacturers were instructed to produce a JN.1-lineage-based COVID-19 vaccine with the preference for the LP.8.1 strain.3
While data was presented showing the importance of COVID-19 vaccination, voting was removed from the agenda, leaving us questioning what to expect for an updated COVID-19 vaccine rollout this fall.
As we navigate a new vaccine environment, several questions are currently at the forefront. One of the main questions is, “What do we do if ACIP’s recommendations contradict evidence-based science?” Many medical organizations such as the American Academy of Pediatrics are committed to science-based recommendations and will be providing their own immunization schedule and guidance. They are also working with insurance companies to include these recommendations for coverage. While that’s a beacon of hope for vaccine coverage, it leaves the question, “What about patients who qualify for Vaccines for Children (VFC)?” If vaccines such as the COVID-19 vaccine are not approved by ACIP, will they be covered by VFC? The VFC program is federally funded and run by the CDC. The CDC provides policy development, operational oversight for the program and technical support. State and local health departments receive funding from CDC to implement and oversee their VFC programs. The VFC program provides coverage for all vaccines recommended by ACIP and approved by the CDC. Approximately 52% of children in the United States qualify for VFC.4
Fall respiratory season will be here soon, and I am hopeful that we’ll have approval for an updated 2025-2026 COVID-19 vaccine at the next ACIP meeting. A tentative ACIP meeting is scheduled for August/September with the dates to be determined.
References:
1. Statement concerning June 2025 ACIP meeting. Voices for Vaccines blog. June 26, 2025. Accessed July 1, 2025. https://www.voicesforvaccines.org/statement-concerning-june-2025-acip-meeting/
2. Work group considerations and clinical considerations for clesrovimab. Maternal and Pediatric RSV Session Coronavirus and Other Respiratory Viruses Division. Presented at the Advisory Committee on Immunization Practices meeting; June 25, 2025. Accessed July 1, 2025. https://www.cdc.gov/acip/downloads/slides-2025-06-25-26/06-MacNeil-Mat-Peds-RSV-508.pdf
3. National Center for Immunization and Respiratory Diseases. Current epidemiology of COVID-19. Coronavirus and Other Respiratory Viruses Division. Presented at the Advisory Committee on Immunization Practices meeting; June 25, 2025. Accessed July 1, 2025. https://www.cdc.gov/acip/downloads/slides-2025-06-25-26/02-MacNeil-COVID-508.pdf
4. About the Vaccines for Children (VFC) Program. Centers for Disease Control and Prevention. June 26, 2024. Accessed July 3, 2025. https://www.cdc.gov/vaccines-for-children/about/index.html