Naltrexone Neuroimaging
The Children’s Mercy Research Institute together with the Divisions of Adolescent Medicine and Clinical Pharmacology are doing a research study to understand brain differences in the reward system of teens and young adults with eating disorders. The study involves a one-time dose of a medication called naltrexone. Naltrexone is used in many conditions in adults and children from autism to eating disorders. It is most known for preventing drug abuse in adults. In patients with eating disorders, it is used to reduce binge eating and purging (such as vomiting). Our previous work showed that naltrexone helps some patients with eating disorders, but not all. We want to understand why some patients respond and others don’t.
*** Participating in research is always completely voluntary.
We are recruiting:
-
Teens and young adults aged 13-21 years
-
Diagnosed with an eating disorder that involves binge eating or purge behaviors like Anorexia Nervosa-Binge/Purge subtype, Bulimia Nervosa or Other Specified Feeding/Eating Disorder
-
Not currently taking naltrexone
-
Stable medication regimen (no changes to current medications or dosages for 4 weeks before the study day)
You cannot participant in the study if you:
-
Have non-removal metal in the body that interferes with MRI (ask if you aren’t sure)
-
Used opioids in the 7 days before the study day
-
Are pregnant
-
Had prior hypersensitivity reaction to naltrexone (e.g., anaphylaxis)
-
One study visit lasting around 10 hours.
-
Answer brief questionnaires and play short computer games (lasting about 30 minutes total)
-
Take one tablet of naltrexone, a medication that temporarily blocks the opioid reward system and is sometimes used in the treatment of eating disorders in teens.
-
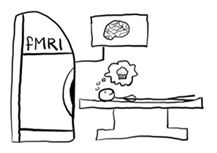
Complete one brain scan called an fMRI (see picture) before taking naltrexone and one brain scan after naltrexone. During the brain scan, the study participant will play a game and view pictures of food.
-
Have blood drawn and urine collected.
Yes. Study participants can be on other medication, just not currently taking naltrexone. It’s okay if the participant has taken this in the past as long as they have been off for at least 4 weeks. Also, participants should have no changes in medication or dose for the 4 weeks before the study day.
We are happy to work with you to try to minimize any disruptions and to schedule visits on days when school is not in session.
The study is one single day, lasting around 10 hours. Participants will arrive at Hoglund Biomedical Imaging Center (on the campus of KUMED) at 7:15 a.m. and the day will end about 5:30 p.m.
Hoglund Biomedical Imaging Center on the campus of KUMED is located at 3805 Eaton St., Kansas City, KS 66103. There is dedicated parking for research participants right in front of the free-standing building.
There are no extra costs for being in this study.
Yes, each study participant will receive $400 for completion of the study day.
- Stephani Stancil, PhD, APRN, Principal Investigator
- Research Coordinators (which include nurses and support staff)
- Other study team members at times.
For more information about this study or to discuss enrollment, please contact the study team at Children's Mercy:
Ileana Cepeda, MPH
Research Associate
iecepeda@cmh.edu
816-652-2291
Stephani Stancil, PhD, APRN
Principal Investigator
slstancil@cmh.edu
To learn more about the study, please fill out the study interest form by either clicking on this link or scanning the QR code with your cell phone or tablet and a member of our study team will contact you within two business days.