Research Information for Families
Clinical research paves the way for new treatments and interventions
Advancing pediatric health lies at the heart of everything we do here at the Children’s Mercy Research Institute (CMRI). This includes an exciting clinical research program to bring the fruits of scientific discovery right to each child who comes to us for care.
Each year, CMRI researchers participate in hundreds of clinical research studies to make discoveries to improve the health and wellbeing of children. The term “clinical research” may involve completing surveys and certain tasks to taking part in studies of new medications and treatments for a disease (called “clinical trials”).
Below are some of the most important things to understand about clinical research before you allow your child to participate in a study.
What is clinical research?
Clinical research — sometimes called medical research — covers a wide range of topics and areas. Because the purpose of clinical research is to gather information on a specific research question, there is no guarantee that your child will directly benefit from taking part in a study. Taking part in a clinical research study or clinical trial helps researchers better understand diseases and possible new treatments and may help children in the future.
Your child can take part in a research study
Your child can take part in research if they have a disease or condition that is being studied or even if they are what is called a “healthy volunteer” — meaning your child does not have the disease or condition.
Your child’s doctor or health care provider, or perhaps another Children’s Mercy staff member who is not directly involved in your child’s care, may tell you about a clinical research study and ask if you would like your child to participate. Or you may explore clinical research opportunities on your own that your child may like to take part in.
Your child has rights as a research volunteer
There are important things to know about clinical research before you decide to allow your child to take part in a study.
- Taking part in a research study is always optional.
- Deciding not to allow your child to take part in a research study will not affect their regular care – now or in the future.
- You can withdraw your child from a study at any time.
- Taking part in a study may involve risks and benefits. The study doctor or member of the study team will go over these with you.
- You should ask any questions you have or share your concerns before you let your child take part in a study.
The difference between research and medical care
Research is how investigators at CMRI and around the world answer questions about diseases and conditions to improve the medical care for children. There is no guarantee your child will directly benefit from taking part in a clinical research study. Everyone who takes part in clinical research studies are carefully monitored by the study team to minimize any risks.
It is important that your child’s doctor know whether he or she is taking part in a research study.
What it means if a trial is “randomized”
If a clinical research study is “randomized,” that means your child will be assigned to a treatment or other group by chance (think of it as flipping a coin). In a randomized study neither you nor the researchers can choose which specific group your child will be assigned to.
For additional information
If you would like additional information about participating in clinical trials, visit the U.S. Department of Health and Human Services website. Their site contains short videos covering topics ranging from "what is medical research?" to "questions to ask before volunteering in clinical trials" and more.
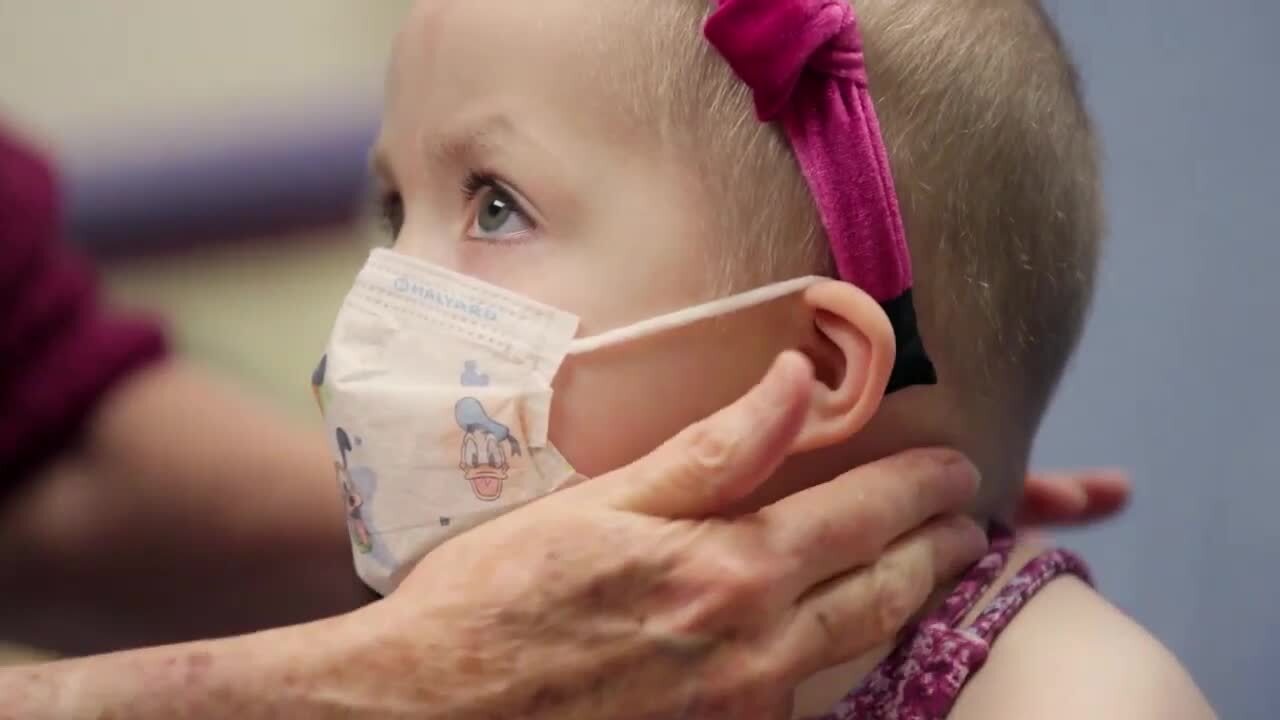
A patient’s perspective
When Elsie just turned three she was diagnosed with acute lymphoblastic leukemia (ALL). Along with her treatment, Elsie's doctor, Maxine Hetherington, MD, brought up the subject of participating in research to her parents. They agreed and Elsie has participated in multiple studies, including Children's Oncology Group clinical trials.
"It is a privilege to be able to affect treatment in a way that hopefully leads to better outcomes for future families going through this," said Elsie's mom.
Clinical research at Children's Mercy
Clinical research at CMRI includes active clinical trials, research projects, clinical registries, programs and more.