The Children’s Mercy Research Institute
The Children’s Mercy Research Institute
Bold science. Built for kids.TM
The Children’s Mercy Research Institute (CMRI) integrates research and clinical care with multidisciplinary teams at the point of care while bringing together nationally recognized expertise in genomic medicine, precision therapeutics, population health and health care innovation.
As a result, we accelerate the development of groundbreaking individualized therapies and treatments that transform the potential of all children, one child at a time.
RESEARCH STORIES
Foundations for the future
Celebrating 10 years of Research at Children’s Mercy Month
THE CHILDREN'S MERCY RESEARCH INSTITUTE NEWS
Dr. Gina Jones Receives Funding to Establish Transition to Adulthood Program for Patients with Movement Disorders
The project is designed to redefine the current Children's Mercy transition program and make it more specific to the needs of patients with movement disorders.
Learn more
5 Questions:
Aswini Betha
The CMRI has a collaborative and innovative team with experts in a wide array of fields to make research happen. Each month, we ask one of our CMRI team members five questions to learn more about their role in research and what motivates them. This month, meet Aswini Betha, PhD, CLP, Senior Director, Technology Transfer & Commercialization, Office of Technology Transfer & Commercialization
Meet our team member
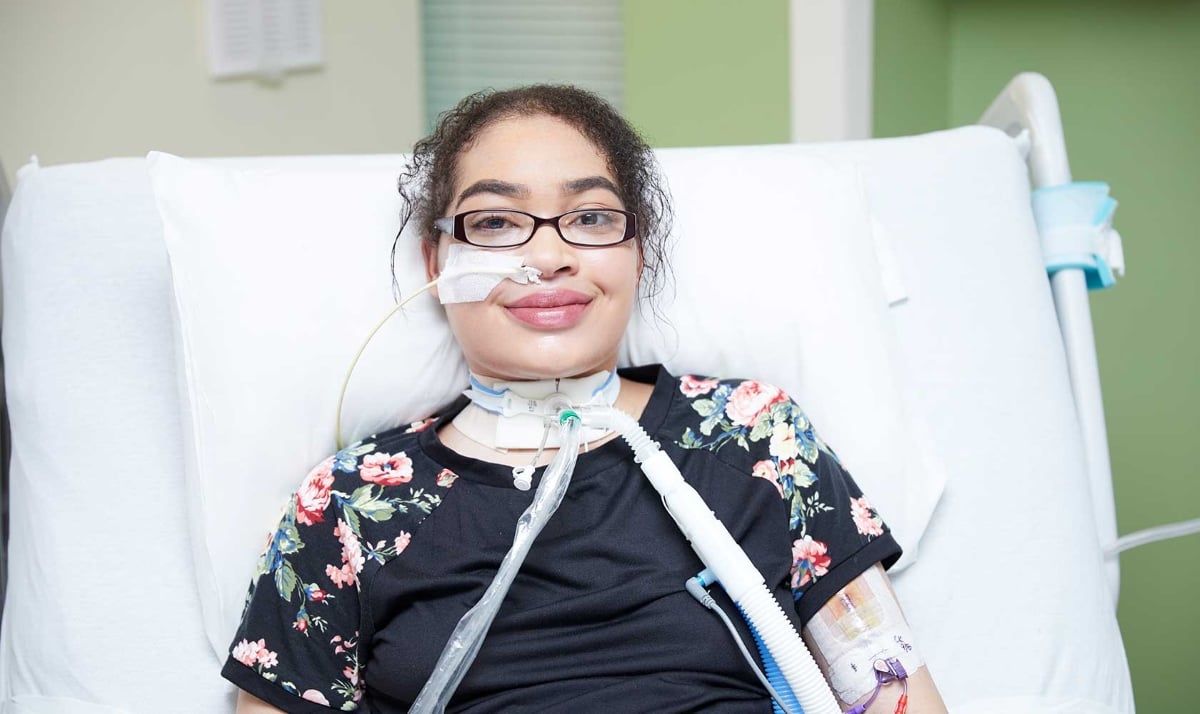
Preventing Deadly Antibiotic Reactions in Children
Two Children’s Mercy doctors are on a crusade to address Bactrim-related deaths.
Learn more and submit your case
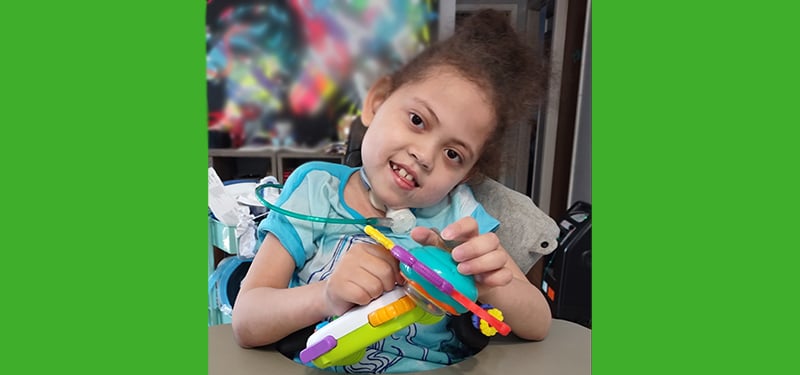
Genomic Answers for Kids
Thousands of genetic diseases affecting children remain undiscovered and untreatable. But hope is on the horizon with Genomic Answers for Kids, a first-of-its-kind pediatric data repository to find novel treatments for pediatric genetic conditions.
Discover more
Researcher Network
Learn about the dynamic research programs and explore potential collaborations with our world-renown investigators.

Join us!
We are looking for volunteers to be a part of the Family and Community Research Advisory Board.
Make a Gift
Donors like you help support the research discoveries of tomorrow.