Scott T. Younger, PhD
Director, Disease Gene Engineering; Associate Professor of Pediatrics, University of Missouri-Kansas City School of Medicine; Research Assistant Professor of Pediatrics, University of Kansas School of Medicine
Full Biography
In January 2025, the prestigious journal, Nature, published a study led by researchers at Children’s Mercy Research Institute (CMRI). A research study conducted by a Kansas City institution had not been featured in Nature for five years.
The publication, “Rapid and scalable personalized ASO screening in patient-derived organoids,” highlights the methods generated by The Younger Laboratory and their significant advancements in technology development. Scott Younger, PhD, Director, Disease Gene Engineering, Genomic Medicine Center, is the principal investigator and leader of The Younger Lab. He expressed deep appreciation for his team and gratitude for the opportunity to publicize their research.
“To build something that is the caliber of what's published in Nature is just incredible and a real testament to the hard work of all the authors that were involved,” said Dr. Younger.
The study has seven co-authors in addition to Dr. Younger: John C. Means, PhD, Assistant Director, Patient Cell Models, Genomic Medicine Center; Anabel L. Martinez Bengochea, PhD, Post-Doctoral Research Scholar, Clinical Pharmacology and Toxicology; Daniel A. Louiselle, MS, Research Associate Masters, Genomic Medicine Center; Jacqelyn M. Nemechek, PhD, Research Scientist, Hematology/Oncology/BMT; John M. Perry, PhD, Doctoral Research Faculty, Hematology/Oncology/BMT; Emily G. Farrow, PhD, CGC, FACMG, Assistant Clinical Laboratory Director, Clinical Genetics and Genomics Laboratory, Pathology and Laboratory Medicine; and Tomi Pastinen, MD, PhD, Vice President, Associate Chief Medical Officer for Clinical and Research Integration, Division Director, Genomic Medicine Center.
To build something that is the caliber of what’s published in Nature is just incredible and a real testament to the hard work of all the authors that were involved.
Benefiting pediatric patients with rare diseases
The Younger Lab developed patient-derived cellular lines from patients in the Genomic Answers for Kids (GA4K) program — a CMRI initiative to create a database for pediatric genomes. The patient cell lines are then differentiated to create organoids — three-dimensional cellular models, also known as mini-organs — in a dish.
Once an organoid is functional, the team can test therapeutics composed of short synthetic nucleic acid sequences — antisense oligonucleotides (ASOs) — tailored to a patient’s own DNA or RNA. ASOs can affect gene expression by binding to select messenger RNAs, where they either block or restore the ability to make certain proteins.
Those who have Duchenne muscular dystrophy (DMD) do not have, or have too little of, the protein dystrophin, which is crucial for muscle health and cardiac function. There are FDA-approved ASOs that increase dystrophin production for a subset of DMD patients who harbor specific genetic mutations in the dystrophin gene. However, Dr. Younger’s team was interested in developing custom tailored, patient-specific ASOs that could be used to treat DMD patients that currently lack therapeutic options.
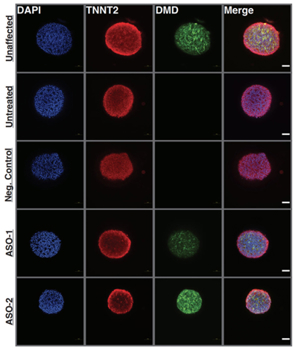
The team used cardiac organoids derived from multiple pediatric DMD patients to evaluate the impact of ASOs on dystrophin expression. The researchers observed that cardiac organoids generated from DMD patients had weak, arrhythmic contraction profiles compared to unaffected control organoids. However, administration of personalized ASOs designed by Dr. Younger’s team were effective at restoring dystrophin expression and improving cardiac function to healthy levels.
A rapid, cost-effective approach
Dr. Younger’s team’s focus was to make this process cost effective, efficient and accessible. Prior to this study, the production of patient-derived cellular models could cost up to $10,000 and required six months to a year to complete. The Younger Lab was able to reduce the cost to around $500 with a faster turnaround time of 2 to 3 weeks. After creating a patient cell line, they can generate fully functional organoids within two months. This process is so efficient that Dr. Means was able to independently generate nearly 400 patient cell lines in approximately six months.
Nature requests information from Dr. Younger
The paper in Nature started organically and went through a lengthy process prior to being published. Dr. Younger unknowingly started the process by submitting an abstract to the American Society of Human Genetics, an organization that hosts an annual international conference on genetics and genomics research. Only the top 1% of submitted abstracts are selected for plenary presentations at the conference. Dr. Younger was among those selected to host a 15-minute plenary talk in November 2022. Unbeknownst to Dr. Younger, an editor for Nature was in the audience, and they requested more information after the presentation. About four months later, Dr. Younger submitted the paper to Nature and underwent three rounds of different review cycles until it was ultimately accepted in November 2024. The article was then published two months later, on Wednesday, Jan. 22, 2025.
Nature is a high-profile, exclusive research journal that publishes a broad range of research topics so for many researchers and scientists, it is a goal to be featured in the publication.
“It brings eyes onto what we're doing and will hopefully have other people see what's going on in the Midwest. The science we're doing can benefit not just us but can benefit other children's hospitals around the country,” said Dr. Means.
Bench-to-bedside research helps Children’s Mercy patients
This fiscal year, after working with DMD patients, The Younger Lab implemented Children’s Mercy’s bedside-to-bench-to-bedside mantra. Jean-Baptiste Le Pichon, MD, PhD, FAAP, Professor of Pediatrics, Interim Director, Neurology, presented a patient with seizures to Dr. Younger’s team. They decided to create a brain organoid to mimic the seizure-like activities. Through this process, they tested a handful of treatments, which eventually ended with a medication that reduced the frequency of seizures.
The Younger Lab also assisted with the Angelman Syndrome Clinic — a medical home that assists patients with the rare genetic disorder, Angelman Syndrome (AS). Over a dozen patient-derived cellular models were created based on patients with AS. The goal of this research is to learn more about AS and find new therapeutic avenues for pediatric patients with this disorder.
A stepping stone for all
Nature shined a spotlight on Dr. Younger’s study, allowing the information to be spread across the country for any scientist or reader. Since being published, The Younger Laboratory has been contacted by multiple institutions, like the University of Pennsylvania and Harvard University, to facilitate their own studies. Dr. Younger’s team is assisting the University of Pennsylvania as they implement this process in their core facilities. Harvard University is also looking to execute the reprogramming portion of this study to generate patient cell lines.
“I just hope that the Nature article is a stepping stone for people to see what can be done to help the patient population,” said Dr. Means. “That there is a way to help patients that doesn't take forever, that can be fast and efficient and that can at least give a patient some type of answer.”
Trained researchers in The Younger Lab have worked with several different organoid models to learn more about rare diseases and ultimately support pediatric patients. Children’s Mercy physicians have access to the platform built by Dr. Younger’s team that makes it easier to tailor treatments for their patients. The platform is scalable and can generate cell models rapidly, with the goal of assisting clinicians by informing therapeutic approaches for patients. The end goal is to implement this platform everywhere, so any child, or adult, can benefit from their technology.
You can read more about our translational research efforts to improve the health and wellbeing of children and so much more in the FY25 Research Annual Report.
