Wise Use of Antibiotics
August 2021
Intersection Between Awareness and Action: Race and Ethnicity in Antimicrobial Stewardship
Author: Elizabeth Monsees, PhD, MBA, RN, CIC, FAPIC | Antibiotic Stewardship Program Manager | Senior PCS | Researcher | Assistant Professor, UMKC School of Medicine

Column Editor: Rana El Feghaly, MD, MSCI | Director, Clinical Services | Director, Outpatient Antibiotic Stewardship Program | Associate Professor of Pediatrics, UMKC School of Medicine
Undoubtably, 2020 was challenging, painful, but also instructive. From the media to large organizations to individual community members, social determinants of health (SDOH) have rightly received focused attention. Considering this within the context of antimicrobial stewardship (AS), there is still much unknown about the influence of SDOH on prescribing among different populations.1
Specific to race and ethnicity, individuals of White race are twice as likely to fill antimicrobial drug prescriptions and among adults, are more likely to be diagnosed with Clostridioides difficile infection (CDI).2,3 However, these data have been difficult to generalize broadly. In the pediatric literature, similar findings exist. White children often receive more antibiotics when compared to Black and Hispanic children.
In a watershed study by Gerber et al., who examined data from an ambulatory network of 222 clinicians from 25 primary care pediatric practices, compared to non-Black children, Black children were:
- Less likely to receive an antibiotic prescription from the same clinician per visit (23.5% vs 29.0%, OR 0.75; 95% CI 0.72-.077).
- Less likely to receive diagnoses that warranted antibiotic treatment (e.g., acute otitis media, acute sinusitis, etc.).
- More likely (34.0% vs 36.9%, OR 0.88; 95% CI 0.82-0.93) to receive guideline-recommended narrow spectrum antimicrobials.4
While these data demonstrate the prescribing looks promising, there was a difference in certain diagnosis rates which could not be explained biologically. For example, when followed over time, Black children developed otitis media at the same rate as non-Black children but were 40% less likely to receive a diagnosis. The authors indicate that it was unclear whether prescribing was truly driven by diagnosis and that more research is needed to explicate unconscious biases and provider perceptions of parent preferences.
A follow-up study found that Black children received less broad spectrum antimicrobials5; this selective focus is congruent with prescribing national treatment recommendations.6 Similar observations were found in antibiotic prescribing in emergency departments for acute respiratory tract infections, where antibiotics are not indicated. Out of 39,445 encounters, non-Hispanic Black and Hispanic children were less likely to receive antibiotics compared to non-Hispanic White children.7 In a recent study, small but statistically significant differences in antibiotic utilization were identified by race and ethnicity among preterm infants.8
Do race and ethnicity influence antibiotic resistance (AR) outcomes?
Observed differences have been reported in penicillin-resistant Streptococcus pneumoniae and invasive community-associated Methicillin-Resistant Staphylococcus aureus (CA-MRSA). In a retrospective cohort study examining patients with community-acquired pneumonia (n=601), penicillin-resistant Streptococcus pneumoniae was more common among Hispanic patients (21.7% vs 0%; p=0.03).9 Using the Centers for Disease Control and Prevention’s Emerging Infections Program surveillance data, invasive CA-MRSA was 4.59 per 100,000 among Whites and 7.60 per 100,000 among Blacks (RR 1.66; 95% CI 1.52-1.80).10 In See et al.’s mediation analyses, socioeconomic variables (e.g., medically unserved areas, education, income, etc.) accounted for 91% of the total effect and there was not a significant association by Black race (RR, 1.05; 95%, 0.92-1.20), thus AR outcomes are related to social factors.10
Studies demonstrating AR outcomes by race are rare and sometimes conflicting making it difficult to prevent “…the next epidemic of inequality.”11 Nadimpalli et al., identify suspected factors contributing to AR morbidity and mortality (see box below). For example, Black or Hispanic/Latinx comprise ~60% of people in U.S. meat-processing plants where contact can increase exposure to zoonotic, resistant pathogens.
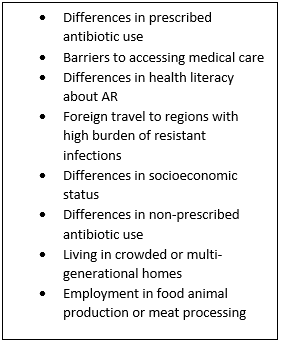
Source: Reference 11 (Nature Medicine online, Figure 1).
How do we drive action?
First, more scholarly activity is needed to develop strategic approaches that help AS programs address SDOH. Increasing evidence supports that emotional, cognitive and social factors influence antibiotic prescribing processes.12-14 Yet, investigation or intervention of AR-related morbidity and mortality rarely includes SDOH data. Authors Fortin-Leung and Wiley outline key AS research questions that can help raise our collective awareness on how race and ethnicity drive prescribing patterns and potential AR outcomes:1
- “Are there certain diseases for which White patients are more likely to be prescribed antibiotics compared to non-White patients?”
- “Are certain antibiotics more likely to be prescribed to Black and Hispanic patients, and are those antibiotics more likely to lead to AR?”
Second, within the AS community, we have work ahead of us to address notable prescribing differences. As sophisticated as our data collection systems are, coupled with our published research, assessment and interventions based on race and ethnicity data are limited. Thus, developing more robust datasets are necessary to inform, guide and promote change.1,11 One recent change in our hospital is applying recommendations from the American Academy of Pediatrics and other professional organizations on the renaming of potentially offensive and racially insensitive terms like replacing “red man syndrome” with vancomycin flushing reaction.15
Finally, as a collective group charged with ensuring the wellbeing of all children, we must be aware of topical emerging literature, accountable for assessing our own practices and reflecting on unrecognized biases, and adaptable to modifying our daily practice.
References:
- Fortin-Leung K, Wiley Z. What about race and ethnicity in antimicrobial stewardship? Infect Control Hosp Epidemiol. 2021:1-2.
- Olesen SW, Grad YH. Racial/ethnic disparities in antimicrobial drug use. United States, 2014-2015. Emerg Infect Dis. 2018;24:2126-8.
- Yang S, Rider BB, Baehr A, Ducoffe AR, Hu DJ. Racial and ethnic disparities in health care-associated Clostridium difficile infections in the United States: state of the science. Am J Infect Control. 2016;44:91-6.
- Gerber JS, Prasad PA, Localio AR, et al. Racial differences in antibiotic prescribing by primary care pediatricians. Pediatrics. 2013.
- Fleming-Dutra KE, Shapiro DJ, Hicks LA, Gerber JS, Hersh AL. Race, otitis media, and antibiotic selection. Pediatrics. 2014.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-73.
- Goyal MK, Johnson TJ, Chamberlain JM, et al. Racial and ethnic differences in antibiotic use for viral illness in emergency departments. Pediatrics. 2017;140:e20170203.
- Flannery DD, Mukhopadhyay S, Jensen EA, et al. Influence of patient characteristics on antibiotic use rates among preterm infants. J Pediatric Infect Dis Soc. 2021;10:97-103.
- Restrepo MI, Velez MI, Serna G, Anzueto A, Mortensen EM. Antimicrobial resistance in Hispanic patients hospitalized in San Antonio, TX with community-acquired pneumonia. Hosp Practice (1995). 2010;38:108-13.
- See I, Wesson P, Gualandi N, et al. Socioeconomic factors explain racial disparities in invasive community-associated Methicillin-Resistant Staphylococcus aureus disease rates. Clin Infect Dis. 2017;64:597-604.
- Nadimpalli ML, Chan CW, Doron S. Antibiotic resistance: a call to action to prevent the next epidemic of inequality. Nature Medicine. 2021;27:187-8.
- Donisi V, Sibani M, Carrara E, et al. Emotional, cognitive and social factors of antimicrobial prescribing: can antimicrobial stewardship intervention be effective without addressing psycho-social factors? J Antimicrob Chemother. 2019;74:2844-7.
- Charani E, Castro-Sanchez E, Sevdalis N, et al. Understanding the determinants of antimicrobial prescribing within hospitals: the role of "prescribing etiquette". Clin Infect Dis. 2013;57:188-96.
- Vaughn VM, Szymczak JE, Newton DW, Fakih MG. Addressing the overuse of cultures to optimize patient care. Ann Intern Med. 2019;171:S73-s4.
- Austin J, Foster A, Empey A. Replace red man syndrome with vancomycin rlushing reaction. Hosp Pediar. 2020;10.

