Wide World of Vaccines
September 2021
COVID-19 Vaccine Potpourri and Booster Confusion

Column Editor: Christopher Harrison, MD | Professor of Pediatrics, UMKC School of Medicine | Clinical Professor of Pediatrics, University of Kansas School of Medicine
News and recently published data:
- Vaccine for 5- to 12-year-olds to be approved within weeks. Pfizer submitted their pediatric data for 5- to 12-year-olds to the FDA for an emergency use authorization (EUA) with approval expected within the next two weeks. We also expect the Moderna vaccine’s EUA for 5- to 12-year-olds within three to four weeks. So, we all need to prepare for questions about 5- to 12-year-olds and consider how to coordinate this age group getting SARS-CoV-2 vaccines in a time frame when we are gearing up for influenza vaccines (Spoiler–they can get both the influenza and SARS-CoV-2 vaccine on the same day if desired). The good news is that expanded pediatric vaccine uptake should reduce the surge of pediatric infections since the school year began, and will reduce risk to teachers/staff who make in-person learning possible.
- Could vaccines help with “long-COVID” symptoms? Among the 22% of surveyed U.S. adults who had COVID-19 infections,1 long-term symptoms (>4 weeks since onset) were more common (65.9%) than in adults with SARS-CoV-2 test-negative respiratory infection (42.9%).1 Both numbers are higher than I expected. I would not have thought that many would have long-haul-like symptoms from non-SARS-CoV-2 infections. Of note, more with long-haul symptoms from SARS-CoV-2 reported improved persisting symptoms after a post-infection COVID-19 vaccine compared to those with non-COVID-19 infections (28.7% versus 15.7%). Perhaps boosting post-infection immune responses hastens recovery in “long COVID.”
- Could vaccine help long-haul COVID by shifting “natural” immune response away from autoantibodies? One group hypothesized that persisting post-COVID-19 symptoms are due to dysregulation of ACE-2 receptors that SARS-CoV-2 uses to enter human cells and replicate. ACE-2 receptors on respiratory epithelium and most bodily organs are required for physiological maintenance and homeostasis. This group showed that those with “long COVID” have higher autoantibody titers against ACE-2 receptors after SARS-CoV-2 infection.2 Treatments that block/reduce ACE-2 autoantibodies could be key to shortening/preventing “long COVID.” Considering the data in Number 1 above, COVID-19 vaccines may shift the immune response away from such autoantibodies to protective SARS-CoV-2 antibodies.
- Moderna better than Pfizer mRNA vaccine? It appears that the Moderna mRNA vaccine is somewhat more immunogenic (more antibodies mostly in those >50 years old - see Figure) than the Pfizer mRNA vaccine, perhaps due to higher mRNA content,3 also correlating to Moderna’s vaccine being more reactogenic. This antibody difference has not made a difference in protection against the original or the Alpha virus, but may offer an advantage against Beta, Delta and Gamma variants.4 That said, post-booster dose, differences shrink and appear unlikely to be clinically important. See Figure.3
- Pfizer booster doses prevent Delta breakthrough infections and are safe in elderly—the Israeli data that Dr. Fauci recently quoted. In a prior column we reported that the Israelis had started a national real-world experiment in response to a large surge of breakthrough Delta variant infections/hospitalizations in May to June 2021 among fully vaccinated persons, particularly among the elderly. They gave over one million Pfizer boosters to >60-year-olds eight to nine months post second dose. Confirmed infections dropped 11.4-fold (95% CI: [10.0, 12.9]), and severe illness dropped >10-fold in booster recipients.5 These data plus other preliminary data that immune response may be less potent and durable in the elderly3 set the stage for how boosters in the U.S. could be initially prioritized beyond the immunocompromised (Spoiler 2–boost the elderly, particularly in nursing homes).
Confusion about boosters. The Biden administration announced boosters (already approved for the immunocompromised) for all starting Sept. 20, 2021, just around the corner.
The case against. Can we roll out big chunks of the limited vaccine supply as boosters in the U.S., a rich country, when the rest of the world is crying for vaccine to use as primary series? The WHO asked repeatedly that rich countries not boost fully vaccinated persons but send doses to developing countries. Previous FDA leaders (now resigned) and some scientists also did not feel the evidence supported boosters for everyone in the U.S.4
The case for. Dr. Fauci quoted the Israeli data (see Number 5 above) that boosters prevent breakthrough infections/hospitalizations especially in the elderly when Delta variants predominate.3 While the large surge in Delta variant U.S. infections, hospitalizations and deaths is in the unvaccinated, highly visible reports reveal at least some breakthrough deaths/hospitalizations in fully vaccinated persons, e.g., post large gatherings and after travel exposures.
Balanced gradual booster plan? History tells us that no vaccine against a respiratory pathogen has ever provided long-term protection with just a primary series. And with variants, this concept is more concerning. Boosters will eventually be needed for everybody. But who really needs a booster now?
At present, the available data on breakthrough hospitalizations/deaths due to the Delta variant (e.g., Israeli data in elderly, San Diego data about health care workers,6 and more severe disease with underlying conditions) tell me that some populations need boosters six to 12 months after the primary series, e.g., now or very soon.
But we can address international needs and U.S. needs by boosting those in the U.S. at highest risk first (those >60 years old and those with underlying conditions) followed soon after (hopefully as vaccine supplies increase) by patient-facing health care workers and frontline essential workers, and only later consider how to boost those at lower risk, much like the initial rollout.
Final thoughts. One vaccinee group needing special consideration are the brave vaccine trial volunteers who are now as much as 10 months post dosing. Data suggest waning protection versus Delta at nine to 12 months. There should be a defined plan for boosting these folks.
I hope U.S. public health decision-makers are not purely reactive, waiting to start boosters only after breakthrough disease reaches compelling numbers. I hope for a selectively proactive U.S. booster approach that does not neglect our responsibility to help less developed countries control their disease burden.
Figure. Effect of Booster Dose of mRNA Vaccine
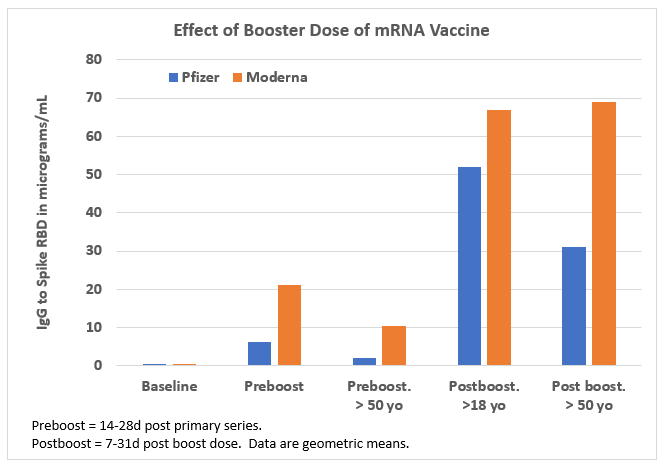
Data source: Reference 3, JAMA Network Open
References:
- Wanga V et al. Long-term symptoms among adults tested for SARS-CoV-2 — United States, January 2020–April 2021. MMWR. Sept. 10, 2021. 70(36):1235-1241.
- Arthur JM, et al. Development of ACE2 autoantibodies after SARS-CoV-2 infection. (2021) PLoS ONE. 16(9): e0257016.
- Richards NE, et al. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine. JAMA Network Open. 2021;4(9):e2124331. doi:10.1001/jamanetworkopen.2021.24331.
- Krause PR, et al. Considerations in boosting COVID-19 vaccine immune responses. Online Sept. 13, 2021. https://doi.org/10.1016/S0140-6736(21)02046-8.
- Bar-On YM, et al. BNT162b2 vaccine booster dose protection: A nationwide study from Israel. medRxiv. Posted Aug. 31, 2021. https://www.medrxiv.org/content/10.1101/2021.08.27.21262679v1.
- Keehner J, et al. Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce. NEJM. Online Sept. 1, 2021. DOI: 10.1056/NEJMc2112981.
